Extraction of vanadium from pet-coke gasification cinder: Part 1 leaching kinetics studies in sulphuric acid medium
Abstract
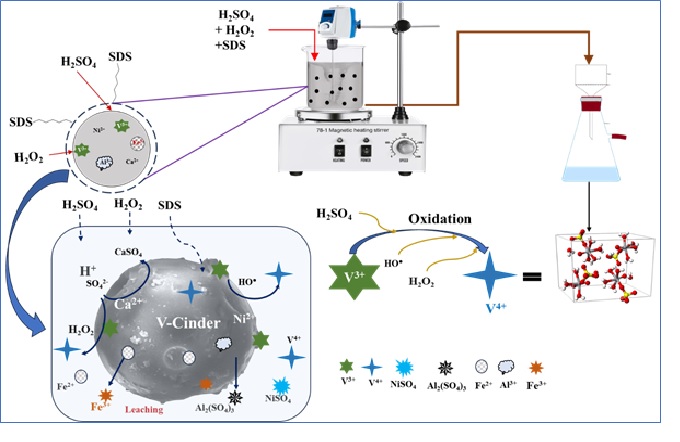
Petcoke cinder is a solid by-product generated during the gasification of petcoke, accounting for three to five percent of the petroleum coke mass. It commonly contains valuable metals like V, Ni, Al, and Fe, highlighting the resource's potential for vanadium extraction. Thus, it can be regarded as an emerging secondary source of vanadium and other critical metals. The conventional pyro-cum-hydrometallurgical process for vanadium recovery relied on alkali roasting, which was not only energy-intensive but also contributed to greenhouse gas emissions. Considering environmental concerns and high energy consumption, the current study embodies a state-of-the-art development of an economically viable process for extracting V from petcoke gasification cinders. This paper examines the method of sulfuric acid-leaching of vanadium from gasification cinders and the effects of various parameters, including acid concentration, temperature, process duration, percentage of H2O2, amount of sodium dodecyl sulfate (SDS), and solid-liquid phase ratios. The maximum vanadium yield of 95.62% was achieved under optimal conditions of sulfuric acid concentration, temperature, time, S/L ratio, and amounts of H2O2 and SDS, which were determined to be 4M, 90°C, 3 hours, 20%, and 6%, respectively. The shrinking surface-controlled model was employed to investigate leaching kinetics. The reaction order concerning sulfuric acid concentrations was found to be 1.29. According to the Arrhenius equation, the evaluated apparent activation energy for leaching was approximately 27.67 kJ/mol, and the empirical equation representing the acid leaching kinetics of vanadium is identified as 1-(1-α)1/3 = kt = (9.261 X 103) [H2SO4]1.2916exp[-27674/RT] T.
References
Bartitto, M., Oni, A.O., Kumar, A., Vanadium recovery from oil sands petcoke fly ash: A comprehensive techno-economic assessment, Waste Management, 194 (2025) 249-257. https://doi.org/10.1016/j.wasman.2025.01.018.
Peng, H., Shang, Q., Chen, R., Leng, Y., Guo, J., Liu, Z., Tao, C., Oxidative Leaching Kinetics of Vanadium from the Vanadium Chromium-Reducing Residue with K2Cr2O7, ACS Omega, 5 (2020) 8777−8783. https://dx.doi.org/10.1021/acsomega.0c00339.
Liu, B., Meng., L., Zheng., S., Li, M., Wang, S., A novel method to extract vanadium from high-grade vanadium slag: non-salt roasting and alkaline leaching, Physicochem. Problem Mineral Processing, 54(3) (2018) 657-667. http://dx.doi.org/10.5277/ppmp1857.
Deng, R., Xiao, H., Xie, Z., Liu, Z., Yu, Q., Chen, G., Tao, C., A novel method for extracting vanadium by low temperature sodium roasting from converter vanadium slag, Chinese Journal of Chemical Engineering, 28 (2020) 2208–2213. https://doi.org/10.1016/j.cjche.2020.03.038.
Liu. L., Kauppinen, T., Tynjala, P., Hu, T., Lassi, U., Water leaching of roasted vanadium slag: Desiliconization and precipitation of ammonium vanadate from vanadium solution, Hydrometallurgy, 215 (2023) 105989. https://doi.org/10.1016/j.hydromet.2022.105989.
Gao, F., Olayiwola, A.U., Liu, B., Wang, S., Du, H., Li, J., Wang, X., Chen, D., Zhang, Y., Review of Vanadium Production Part I: Primary Resources, Mineral Processing and Extractive Metallurgy Review, 43(4), (2022) 466–488. https://doi.org/10.1080/08827508.2021.1883013.
Lu, G., Zhang, T., Zhang, G., Zhang, W., Zhang, Y., Dou, Z., Wang, L., Wang, Y., Xie, G., Process and Kinetic Assessment of Vanadium Extraction from Vanadium Slag Using Calcification Roasting and Sodium Carbonate Leaching, JOM 71 (2019) 4600-4607. https://doi.org/10.1007/s11837-019-03672-9.
Petranikova, M., Tkaczyk, A., Bartl, A., Amato, A., Lapkovskis, V., Tunsu, C., Vanadium sustainability in the context of innovative recycling and sourcing development, Waste Management, 113 (2020) 521-544. http://dx.doi.org/10.1016/j.wasman.2020.04.007.
Kurniawan, K., Sookyung, K., Mooki, B., Hyunju, L., Jae-chun, L., A Review on the Metallurgical Recycling Process of Vanadium from Secondary Resources, Mineral Processing and Extractive Metallurgy Review, 45(7) 2023 697-727. https://doi.org/10.1080/08827508.2023.2243007.
Jammulamadaka, H. and Pisupati, S. V., A Critical Review of Extraction Methods for Vanadium from Petcoke Ash, Fuels, 4 (2023) 58–74. https://doi.org/10.3390/fuels4010005.
Vishnyakov, A., Vanadium and Nickel Recovery from the Products of Heavy Petroleum Feedstock Processing: A Review Metals, 13 (2023) 1031. https://doi.org/10.3390/met13061031.
Emami, S.A. and Kelishadi, M.R., A Kinetic Study of Vanadium Dissolution During Acetic Acid Leaching of Steel Making Converter Slag, Journal of Advanced Materials and Processing, 8(4) (2020) 35-44. http://dorl.net/dor/20.1001.1.2322388.2020.8.4.4.9.
Zhang, Z., Sun, C., Li, H., Yin, W., Zhou, J., Blank roasting kinetics of illite type vanadium bearing stone coal, Journal of Material Research and Technology, 9 (2020) 7363-7369. https://doi.org/10.1016/j.jmrt.2020.05.010.
Bakker, A., Seleman, M.M.El-S.S. Ahmed,M.M.Z., Harb, S., Goren, S., Howsawi, E., Recovery of vanadium and nickel from heavy oil fly ash (HOFA): a critical review RSC Advances, 13 (2023) 6327. https://doi.org/10.1039/D3RA00289F.
Liu, S., Chen, Y., Yu, S., Zhang, D., Xie, G., Rapid Vanadium Extraction from Roasted Vanadium Steel Slag via a H2SO4−H2O2 System: Process and Mechanism. ACS Omega, 7 (2022) 25580−25589. https://doi.org/10.1021/acsomega.2c02744.
Kondrasheva, N. K., Rudko, V.A., Lukonin, R.E., Derkunskii, I.O., The influence of leaching parameters on the extraction of vanadium from petroleum coke, Petroleum Science and Technology, 37(12) (2019) 1455-1462. https://doi.org/10.1080/10916466.2019.1590406.
In-Hyeok, C., Hye-Rim, K., Gyeonghye, M., Jyothi, R. K., Jin-Young, L., Spent V2O5-WO3/TiO2 catalyst processing for valuable metals by soda roasting-water leaching, (2017). https://doi.org/10.1016/j.hydromet.2017.12.010.
Jung, M. and Mishra, B., Vanadium Recovery from Oil Fly Ash by Carbon Removal and Roast-Leach Process, The Minerals, Metals & Materials Society, 70(2) (2018) 168-172. https://doi.org/10.1007/s11837-017-2653-7.
Zhang, C., Zhou, Q., Shen., Liu., G., Wang., Y., Qi, T., Peng, Z., Li, X., Efficient and sustainable process for separating and recovering vanadium from Bayer vanadium sludge using PbSO4 as selective precipitant, Separation and Purification Technology, 341 (2014)126719. https://doi.org/10.1016/j.seppur.2024.126719.
Yang, Z., Li, H-Y., Yin, X-C., Yan, Z-M., Yan, X-M., Xie, B., Leaching kinetics of calcification roasted vanadium slag with high CaO content by sulfuric acid, International Journal of Mineral Processing, 133 (2014) 105–111. http://dx.doi.org/10.1016/j.minpro.2014.10.011.
Zhang, S., Li, G., Xiao, R., Luo, J., Yi, L., Rao, M., Extraction of vanadium from low-vanadium grade magnetite concentrate pellets with sodium salt. Journal of material research and technology, 15 (2021) 5712-5722. https://doi.org/10.1016/j.jmrt.2021.11.039.
Cai, Z. and Zhang, Y., Phase transformations of vanadium recovery from refractory stone coal by novel NaOH molten roasting and water leaching technology, RSC Advances, 7 (2017) 36917. https://doi.org/10.1039/C7RA04741J.
Cai, Z., Zhang, Y., Liu, T., Huang, J., 2016. Mechanisms of Vanadium Recovery from Stone Coal by Novel BaCO3/CaO Composite Additive Roasting and Acid Leaching Technology, Minerals, 6 (2016) 26. https://doi.org/10.3390/min6020026.
Xu, Z., Tang, K., Chen, Y., Zhang, Q., Du., J., Liu, Z., Tao, C., Promoting the Calcified Roasting of Vanadium Slag Based on the CeO2 Catalytic Oxidation Mechanism, ACS Omega. 9 (2024) 16810−16819. https://doi.org/10.1021/acsomega.4c01211.
Maria, K., Toni, K., Tao, H., Pekka, T., Rita, K., Ulla, L., Janne, P., Two-stage leaching of calcium and vanadium from high calcium steelmaking slag, Environmental Technology, 45(27) (2024) 5966-5981. https://doi.org/10.1080/09593330.2024.2316671.
Wang, Z., Peng, Z., Li, Y., Zhu, Y., Xie, K., Selective sulfuric acid cyclic leaching of vanadium from the calcification roasting pellets of vanadium titanomagnetite, Journal of Materials Research and Technology, 23 (2023) 778-790. https://doi.org/10.1016/j.jmrt.2023.01.046.
Authors retain copyright of the published papers and grant to the publisher the non-exclusive right to publish the article, to be cited as its original publisher in case of reuse, and to distribute it in all forms and media.
The Author(s) warrant that their manuscript is their original work that has not been published before; that it is not under consideration for publication elsewhere; and that its publication has been approved by all co-authors, if any, as well as tacitly or explicitly by the responsible authorities at the institution where the work was carried out. The Author(s) affirm that the article contains no unfounded or unlawful statements and does not violate the rights of others. The author(s) also affirm that they hold no conflict of interest that may affect the integrity of the Manuscript and the validity of the findings presented in it. The Corresponding author, as the signing author, warrants that he/she has full power to make this grant on behalf of the Author(s). Any software contained in the Supplemental Materials is free from viruses, contaminants or worms.The published articles will be distributed under the Creative Commons Attribution ShareAlike 4.0 International license (CC BY-SA).
Authors are permitted to deposit publisher's version (PDF) of their work in an institutional repository, subject-based repository, author's personal website (including social networking sites, such as ResearchGate, Academia.edu, etc.), and/or departmental website at any time after publication.
Upon receiving the proofs, the Author(s) agree to promptly check the proofs carefully, correct any typographical errors, and authorize the publication of the corrected proofs.
The Corresponding author agrees to inform his/her co-authors, of any of the above terms.
